Laboratory system management Sistema di Gestione Laboratorio
LIMS
A Laboratory Management System allows organizing and managing all phases of Test campaigns.
From Request and planning, to sample management, bench and equipment maintenance, and instrument calibration.
It prepares test sequences and monitors progress with dashboards.
Digital Transformation
EFFICIENT LABORATORY
ORGANIZED
CONNECTED
ConnessoThe integration of a dedicated system for laboratory organization. A LIMS, or Laboratory Information Management System, is a comprehensive software solution designed to optimize and streamline laboratory management.
QX-LIMS offers advanced features for request management, sample traceability, test execution, and reporting, ensuring compliance with regulations.
- Test Request
- Test Planning
- Test Monitoring
- Test Sequence Editor (TBD)
- API LabVIEW
- Tools Maintenance
- Gage Calibration
- Sample Management
- Test Report
Test Request
The test request, within its workflow, allows for the request and all phases of organization and planning, up to the closure with the test results and the confirmation and validation of the results.
List of Test
A detailed list of tests, complete with associated resources and equipment. Laboratory resources are enabled based on a competency matrix for individual tests.
Asset and Maintenance
The maintenance software is active, allowing you to manage assets, test machines, and maintenance activities.
Calibration Management System
Measurement tools under control, organize internal or external calibrations, track calibrations.
DOCUMENTS
From procedures to test reports, everything is controlled to ensure that only authenticated and approved versions are used.
API LabVIEW
For System Integrators, a series of APIs are available and tested to integrate your automated tests into LabVIEW. Through these APIs, we can ensure automation and direct data acquisition from instruments.
Test Request Flow
TRQ
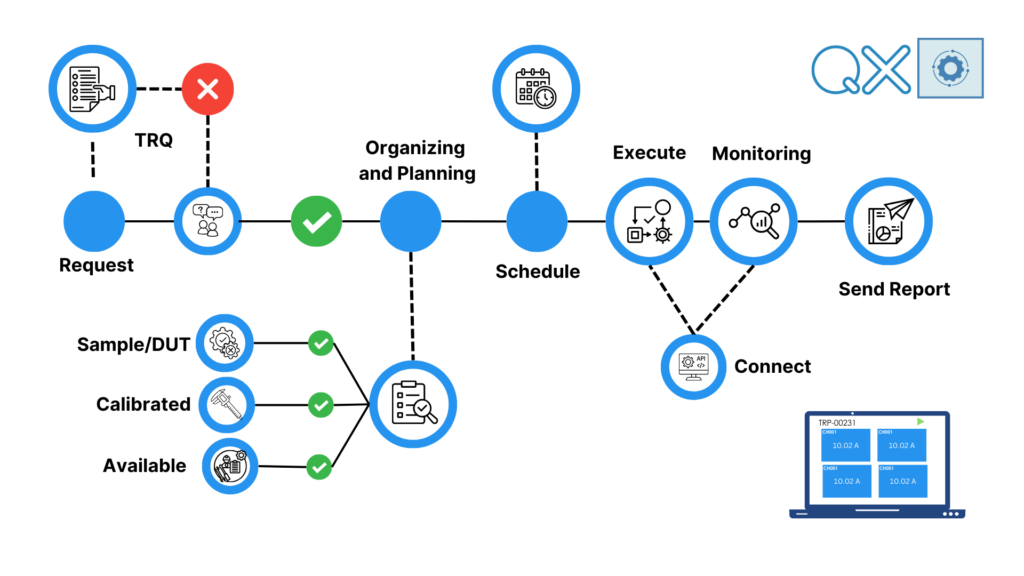
Automation, standardization, and ease of use are at the heart of this process.
Anyone can submit a test request quickly and easily. Negotiation on the test request allows defining with the summoned manager whether to record an order for tests or not.
Once the laboratory manager takes charge, they verify the testing capability, including calibrated instruments, available and updated methods, whether the samples are prototypes or from production, and the definition of DUTs (Devices Under Test) to be tested.
Test plan scheduling, test execution, and monitoring follow.
Closing the Test Request Queue (TRQ) and sending out Test Reports complete the process.
